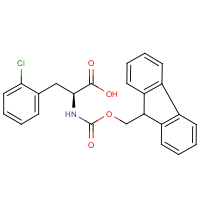
Fmoc-L-Lysine-(Boc), 5 g, CAS No. 71989-26-9 | Fluorenylmethylene / Fmoc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International
Solvent-controlled self-assembly of Fmoc protected aliphatic amino acids - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP05938J

Fmoc-OASUD: A new reagent for the preparation of Fmoc-amino acids free from impurities resulting from Lossen rearrangement - ScienceDirect
Fmoc-L-Proline, 50 g, CAS No. 71989-31-6 | Fluorenylmethylene / Fmoc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications

Molecules | Free Full-Text | Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine?

An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media - Green Chemistry (RSC Publishing)

Arylboronate Ester Protected Amino Acids as Orthogonal Building Blocks for Fmoc Solid‐Phase Peptide Synthesis - Liu - 2017 - European Journal of Organic Chemistry - Wiley Online Library

Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent

Development of Fmoc-Protected Bis-Amino Acids toward Automated Synthesis of Highly Functionalized Spiroligomers | Organic Letters

Synthesis of Fmoc-protected 4-N,N,-dimethylaminophthalimidoalanine (1)... | Download Scientific Diagram




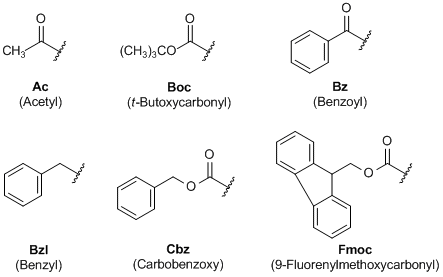

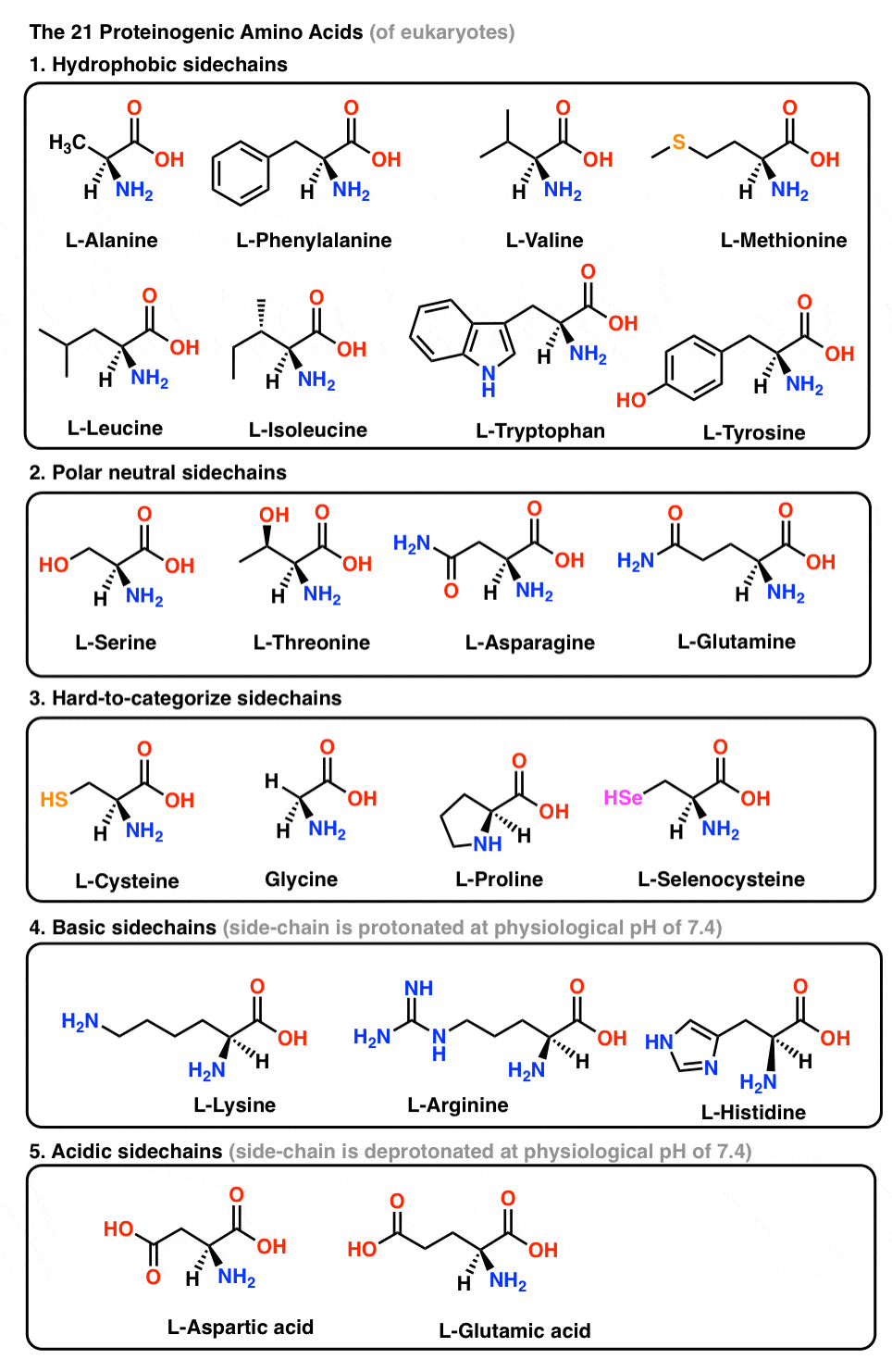
![Fmoc-Amino Acids [N-Protected Amino Acids] | TCI AMERICA Fmoc-Amino Acids [N-Protected Amino Acids] | TCI AMERICA](https://www.tcichemicals.com/medias/B3669.jpg?context=bWFzdGVyfHJvb3R8MTYwMzl8aW1hZ2UvanBlZ3xoMmMvaDk0LzkwODY4NzA5Nzg1OTAvQjM2NjkuanBnfDZjMWQ5ZWRhMzRjODI0Y2JhNmY4MGE0MDFmOTM1NGZiZDk2ZmYxZGYzNzE0MDEyN2JlMWQ5MzI1NzU1ODg0ZDk)